Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -synuclein related to propensity to amyloid fibril formation
-synuclein related to propensity to amyloid fibril formationFujiwara, Satoru*; Kono, Fumiaki*; Matsuo, Tatsuhito*; Sugimoto, Yasunobu*; Matsumoto, Tomoharu*; Narita, Tetsuhiro*; Shibata, Kaoru
Journal of Molecular Biology, 431(17), p.3229 - 3245, 2019/08
Times Cited Count:12 Percentile:49.22(Biochemistry & Molecular Biology) -synuclein (
-synuclein ( Syn) is an intrinsically disordered protein (IDP) with unknown function.
Syn) is an intrinsically disordered protein (IDP) with unknown function.  Syn is known to form amyloid fibrils, which are implicated with the pathogenesis of Parkinson's disease and other synucleinopathies. Elucidating the mechanism of fibril formation of
Syn is known to form amyloid fibrils, which are implicated with the pathogenesis of Parkinson's disease and other synucleinopathies. Elucidating the mechanism of fibril formation of  Syn is therefore important for understanding the mechanism of the pathogenesis of these diseases. Here, using the quasielastic neutron scattering (QENS) and small-angle X-ray scattering (SAXS) techniques, we investigated the dynamic and structural properties of
Syn is therefore important for understanding the mechanism of the pathogenesis of these diseases. Here, using the quasielastic neutron scattering (QENS) and small-angle X-ray scattering (SAXS) techniques, we investigated the dynamic and structural properties of  Syn. These results imply that fibril formation of
Syn. These results imply that fibril formation of  Syn requires not only the enhanced local motions but also the segmental motions such that the proper inter-molecular interactions are possible.
Syn requires not only the enhanced local motions but also the segmental motions such that the proper inter-molecular interactions are possible.

 reductase by X-ray crystallography; New insights into regulation of efficient electron transfer
reductase by X-ray crystallography; New insights into regulation of efficient electron transferYamada, Mitsugu*; Tamada, Taro; Takeda, Kazuki*; Matsumoto, Fumiko*; Ono, Hiraku*; Kosugi, Masayuki*; Takaba, Kiyofumi*; Shoyama, Yoshinari*; Kimura, Shigenobu*; Kuroki, Ryota; et al.
Journal of Molecular Biology, 425(22), p.4295 - 4306, 2013/11
Times Cited Count:21 Percentile:50.76(Biochemistry & Molecular Biology)NADH-Cytochrome 
 reductase (b5R), a flavoprotein consisting of NADH and flavin adenine dinucleotide (FAD) binding domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome
reductase (b5R), a flavoprotein consisting of NADH and flavin adenine dinucleotide (FAD) binding domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome 
 (Cb5). The crystal structures of both the fully reduced form and the oxidized form of porcine liver b5R were determined. In the reduced b5R structure determined at 1.68
(Cb5). The crystal structures of both the fully reduced form and the oxidized form of porcine liver b5R were determined. In the reduced b5R structure determined at 1.68 resolution, the relative configuration of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent-accessible surface area of FAD and created a new hydrogen-bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat as in the oxidized form and stacked together with the nicotinamide ring of NAD
resolution, the relative configuration of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent-accessible surface area of FAD and created a new hydrogen-bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat as in the oxidized form and stacked together with the nicotinamide ring of NAD . Determination of the oxidized b5R structure, including the hydrogen atoms, determined at 0.78
. Determination of the oxidized b5R structure, including the hydrogen atoms, determined at 0.78 resolution revealed the details of a hydrogen-bonding network from the N5 atom of FAD to His49 via Thr66. Both of the reduced and oxidized b5R structures explain how backflow in this catalytic cycle is prevented and the transfer of electrons to one-electron acceptors such as Cb5 is accelerated. Furthermore, crystallographic analysis by the cryo-trapping method suggests that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.
resolution revealed the details of a hydrogen-bonding network from the N5 atom of FAD to His49 via Thr66. Both of the reduced and oxidized b5R structures explain how backflow in this catalytic cycle is prevented and the transfer of electrons to one-electron acceptors such as Cb5 is accelerated. Furthermore, crystallographic analysis by the cryo-trapping method suggests that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.
 1-tetrahydrocannabinolic acid (THCA) synthase, the enzyme controlling the psychoactivity of
1-tetrahydrocannabinolic acid (THCA) synthase, the enzyme controlling the psychoactivity of 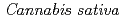
Shoyama, Yoshinari*; Tamada, Taro; Kurihara, Kazuo; Takeuchi, Ayako*; Taura, Futoshi*; Arai, Shigeki; Blaber, M.*; Shoyama, Yukihiro*; Morimoto, Satoshi*; Kuroki, Ryota
Journal of Molecular Biology, 423(1), p.96 - 105, 2012/10
Times Cited Count:82 Percentile:89.24(Biochemistry & Molecular Biology) 1-tetrahydrocannabinolic acid (THCA) synthase catalyzes the oxidative cyclization of cannabigerolic acid (CBGA) into THCA, the precursor of the primary psychoactive agent
1-tetrahydrocannabinolic acid (THCA) synthase catalyzes the oxidative cyclization of cannabigerolic acid (CBGA) into THCA, the precursor of the primary psychoactive agent  1-tetrahydrocannabinol in
1-tetrahydrocannabinol in 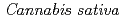 . The structure-function relationship of THCA synthase was investigated by X-ray structure determination (2.75
. The structure-function relationship of THCA synthase was investigated by X-ray structure determination (2.75  resolution) and mutational analysis. Specific amino acid residues were identified in the active site of THCA synthase that are involved in the oxidative cyclization of the CBGA substrate.
resolution) and mutational analysis. Specific amino acid residues were identified in the active site of THCA synthase that are involved in the oxidative cyclization of the CBGA substrate.
Fujiwara, Satoru; Matsumoto, Fumiko
Journal of Molecular Biology, 367(1), p.16 - 24, 2007/03
Times Cited Count:6 Percentile:10.29(Biochemistry & Molecular Biology)In striated muscles, contraction is regulated by the thin filament-based proteins, troponin consisting of three subunits (TnC, TnI, and TnT), and tropomyosin. Knowledge of in situ structures of these proteins is indispensable for elucidating this Ca -sensitive regulatory mechanism. We found from neutron scattering experiments that TnC within the thin filaments assumes extended dumbbell-like structures and moves toward the filament axis by binding of Ca
-sensitive regulatory mechanism. We found from neutron scattering experiments that TnC within the thin filaments assumes extended dumbbell-like structures and moves toward the filament axis by binding of Ca . Here, in order to obtain more detailed in situ structural information of TnC, neutron fiber diffraction measurements were performed. Neutron fiber diffraction patterns were obtained from the oriented samples of native thin filaments and the thin filaments containing deuterated TnC in the absence and presence of Ca
. Here, in order to obtain more detailed in situ structural information of TnC, neutron fiber diffraction measurements were performed. Neutron fiber diffraction patterns were obtained from the oriented samples of native thin filaments and the thin filaments containing deuterated TnC in the absence and presence of Ca . Analysis of the meridional reflections due to Tn-complex with aid of model calculation showed that the angle between the thin filament axis and the long axis of TnC was estimated to be 67(
. Analysis of the meridional reflections due to Tn-complex with aid of model calculation showed that the angle between the thin filament axis and the long axis of TnC was estimated to be 67( 7)
7) and 49(
and 49( 17)
17) , in the absence and presence of Ca
, in the absence and presence of Ca , respectively, suggesting that TnC, which assumes orientations rather perpendicular to the filament axis in the absence of Ca
, respectively, suggesting that TnC, which assumes orientations rather perpendicular to the filament axis in the absence of Ca , tilts toward the filament axis and the orientational and positional disorder increases by binding Ca
, tilts toward the filament axis and the orientational and positional disorder increases by binding Ca . It also showed that the relative position of the TnC moved by about 22
. It also showed that the relative position of the TnC moved by about 22 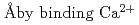 , and this apparent movement was concomitant with the movements of other Tn-subunits. This implies that by binding Ca
, and this apparent movement was concomitant with the movements of other Tn-subunits. This implies that by binding Ca , significant structural rearrangements of Tn-subunits occur.
, significant structural rearrangements of Tn-subunits occur.
Matsumoto, Fumiko*; Makino, Koji*; Maeda, Kayo*; Patzelt, H.*; Maeda, Yuichiro*; Fujiwara, Satoru
Journal of Molecular Biology, 342(4), p.1209 - 1221, 2004/09
Times Cited Count:19 Percentile:29.79(Biochemistry & Molecular Biology)Regulation of skeletal and cardiac muscle contraction is associated with the thin filament-based proteins, troponin C (TnC), TnI, TnT, tropomyosin, and actin. Knowledge of  structures of these proteins is indispensable for elucidating the molecular mechanism of this Ca
structures of these proteins is indispensable for elucidating the molecular mechanism of this Ca -sensitive regulation. Here the structure of TnC within the thin filaments was investigated with neutron scattering, combined with selective deuteration and the contrast matching technique. Deuterated TnC was prepared, reconstituted into the native thin filaments, and neutron scattering patterns of these reconstituted thin filaments containing deuterated TnC were measured under the condition where non-deuterated components were rendered 'invisible' to neutrons. The obtained scattering curves arising only from deuterated TnC were analyzed by model calculations using the Monte Carlo method. The results showed that upon binding of Ca
-sensitive regulation. Here the structure of TnC within the thin filaments was investigated with neutron scattering, combined with selective deuteration and the contrast matching technique. Deuterated TnC was prepared, reconstituted into the native thin filaments, and neutron scattering patterns of these reconstituted thin filaments containing deuterated TnC were measured under the condition where non-deuterated components were rendered 'invisible' to neutrons. The obtained scattering curves arising only from deuterated TnC were analyzed by model calculations using the Monte Carlo method. The results showed that upon binding of Ca ,
,  radius of gyration of TnC changed from 23 AA to 24 AA , and the radial position of TnC within the thin filament changed from 53 AA to 49 AA .
radius of gyration of TnC changed from 23 AA to 24 AA , and the radial position of TnC within the thin filament changed from 53 AA to 49 AA .
Basu, G.*; Sivanesan, D.*; Kawabata, Takeshi*; Go, Nobuhiro
Journal of Molecular Biology, 342(3), p.1053 - 1066, 2004/09
Times Cited Count:23 Percentile:35.53(Biochemistry & Molecular Biology)no abstracts in English
Fujiwara, Satoru; Matsumoto, Fumiko*; Yonezawa, Yasushige*
Journal of Molecular Biology, 331(1), p.21 - 28, 2003/08
Times Cited Count:45 Percentile:59.1(Biochemistry & Molecular Biology)The kinetic process of the fibril formation of hen egg white lysozyme (HEWL) in 90% ethanol in various salt concentrations has been investigated with time-resolved neutron scattering. It was shown that by addition of NaCl in a range between 0.3 mM and 1.0 mM, gelation occurred, and this gelation proceed through a two-step process; the lateral association of the protofilaments formed by HEWL, followed by the cross-linking of these fibrils formed. Both the structures of the fibrils and the rate of the gelation depended on NaCl concentration. Above 2 mM NaCl, precipitation occurred because of the formation of amorphous aggregates. Sensitivity of the aggregated structures to salt concentration suggests that electrostatic interaction plays an essential role in the formation of these structures. The structural diversity both in the fibrils and the aggregated structures of the fibrils can be interpreted in terms of the difference in the degree of the electrostatic shielding at different salt concentrations.
Yonezawa, Yasushige*; Tanaka, Shimpei*; Kubota, Tomomi*; Wakabayashi, Katsuzo*; Yutani, Katsuhide*; Fujiwara, Satoru
Journal of Molecular Biology, 323(2), p.237 - 251, 2002/10
Times Cited Count:76 Percentile:74.78(Biochemistry & Molecular Biology)It is known that hen egg white lysozyme (HEWL) forms amyloid fibrils in highly concentrated ethanol solutions. In order to gain an insight into the mechanism of the amyloid fibril formation, the structures of HEWL in solutions of various protein and ethanol concentrations were investigated with small-angle X-ray and neutron scattering. It was shown that the structural states of HEWL were distinguished as the monomer state, the state of the dimer formation, the state of the protofilament formation, the protofilament state, and the state towards the formation of the amyloid fibrils. Circular dichroism measurements showed that the large changes in the secondary structures of HEWL occurred during the dimer formation. Structural characterization showed that the dimers had an elongated shape, the protofilaments were formed by stacking of the dimers with their long axis (nearly) perpendicular to the protofilament axis, and the changes of the structural states towards the amyloid fibril formation occurred via lateral association of the protofilaments.